Research Interests
The goal of our research is to use computational tools to unravel and understand physico-chemical rules behind biological processes.
We develop new, cutting-edge computational methods that allow us to predict and analyze the interactions of small organic molecules (either synthetic or from natural sources) with biological macromolecular structures such as proteins and DNA/RNA.
We apply these tools in combination with other molecular modeling techniques to investigate and interpret the nature of biological events from a mechanistic and thermodynamic perspective, and rapidly explore the chemical space to identify molecular modulators of therapeutically relevant targets. We have a number of collaborations within Scripps Research as well as with researchers from other academic institutions and pharmaceutical industries.
AutoDock Suite
We develop computational tools for structure based drug design, especially molecular docking software. AutoDock and AutoDock Vina are open-source software and part of the most widely used docking suite, previously developed in the Molecular Graphics Lab, now CCSB. We are developing the next generation of docking engines and the virtual screening tools, designing improved energy potentials, better Free Energy of Binding estimates, and new protocols for drug design.
The goal is to make docking more accurate and useful by making it faster and more chemically meaningful. We are improving the description of the chemical, physical and thermodynamic terms to include torsional preferences of rotatable bonds, a more precise description of non-covalent interactions, and, most importantly, of solvation effects. For this, we look up to modern force fields, electronic structure methods, and molecular dynamics simulations. At the same time, in conjunction with GPU computation, we explore new search algorithms to navigate the energy landscape.

Relevant publications
-
Charting hydrogen bond anisotropy (JCTC 2020)
-
Computational protein–ligand docking and virtual drug screening with the AutoDock suite (Nat.Protoc. 2016)
-
A force field with discrete displaceable waters and desolvation entropy for hydrated ligand docking (JMedChem 2012)
-
AutoDock4Zn: An improved AutoDock force field for small-molecule docking to Zinc metalloproteins (JChemInfMod 2014)
-
AutoDock Bias: Improving binding mode prediction and virtual screening using known protein–ligand interactions (Bioinformatics 2019)
-
Accelerating AutoDock4 with GPUs and gradient-based local search (2019)
-
D3R Grand Challenge 4: Prospective pose prediction of BACE1 ligands with AutoDock-GPU (2019)
Partners
The development of our code is performed in collaboration with hardware and software industry partners.



Covalent inhibitor high-throughput screening
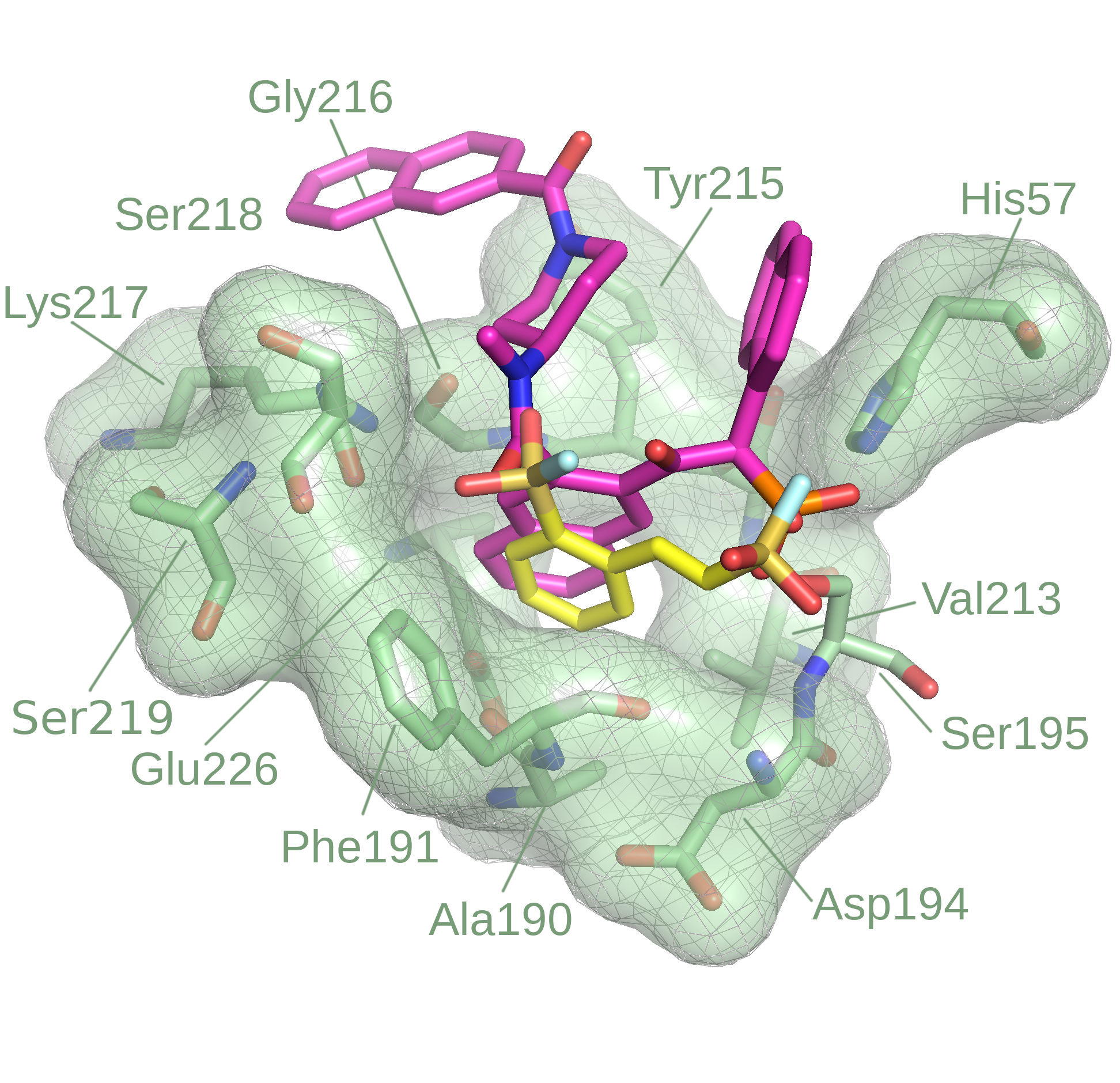
Modeling of covalent binders is non-trivial, especially in a high-throughput fashion. Several covalent methods are now available but the majority of these approaches is effective when information about the target structures is known (i.e. covalent binding site).
Driven by the need to overcome this barrier, we are designing the Reactive Docking method, an approach that allows prospective prediction of covalent binding sites on a protein, through the simulation of the reaction event between the covalent residue and the warhead of the ligand. Furthermore, Reactive Docking allows to model different residues and various types of warheads.
This method was successfully applied to several targets and it is suitable for the HTVS of covalent modifiers for the discovery of new covalent binding sites on different proteins.
Relevant publications
-
Proteome-wide covalent ligand discovery in native biological systems (Nature 2016)
-
“Inverse drug discovery” strategy to identify proteins that are targeted by latent electrophiles as exemplified by aryl fluorosulfates (JACS 2018)
-
Covalent docking using AutoDock: Two‐point attractor and flexible side chain methods (ProtSci 2018)
-
SuFEx-enabled, agnostic discovery of covalent inhibitors of human neutrophil elastase (PNAS 2019)
-
Global profiling of lysine reactivity and ligandability in the human proteome (NatChem 2017)
-
Integrative X-ray structure and molecular modeling for the rationalization of procaspase-8 inhibitor potency and selectivity (ACS ChemBio 2020)
-
Expedited mapping of the ligandable proteome using fully functionalized enantiomeric probe pairs (NatChem 2019)
HIV-1 life cycle
The search for drugs that can interfere with different steps of the HIV-1 life cycle is still a subject of great interest, since the currently available drugs are not able to eradicate the infection. We are targeting the Capsid protein of the HIV-1 by implementing a strategy based on conventional and reactive docking for the Virtual screening of small molecules that could modulate the viral assembly. Reactive Docking approach is being used to screen small molecules with a SuFEx warhead, that could tightly bind to the identified pocket.
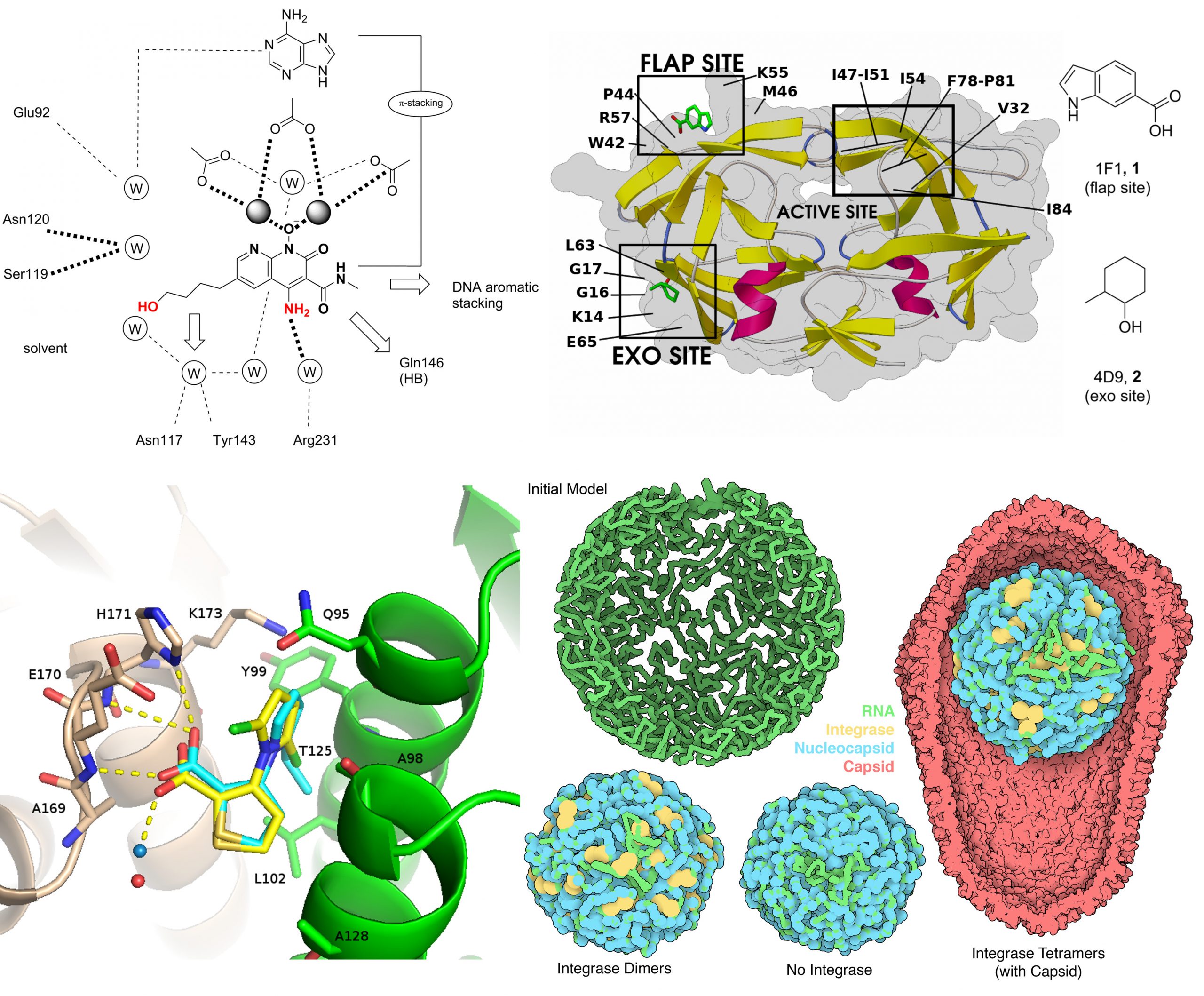
The Forli lab is part of the HIV Interaction in Viral Evolution Center (HIVE):
Relevant publications
-
Structural basis for strand-transfer inhibitor binding to HIV intasomes (Science, 2020)
-
Integrative modeling of the HIV-1 ribonucleoprotein complex (PLoS Comput.Bio., 2019)
-
A new class of allosteric HIV-1 integrase inhibitors identified by crystallographic fragment screening of the catalytic core domain (JBC, 2016)
-
Distinguishing binders from false positives by free energy calculations: Fragment screening against the flap site of HIV protease (J.Phys.Chem.B., 2015)
-
Blind prediction of HIV integrase binding from the SAMPL4 challenge (JCAMD 2014)
-
Crystallographic fragment‐based drug discovery: Use of a brominated fragment library targeting HIV protease (Chem.Biol.Drug Des., 2014)
-
3D molecular models of whole HIV-1 virions generated with cellPACK (Faraday Discuss., 2014)
-
Novel intersubunit interaction critical for HIV-1 core assembly defines a potentially targetable inhibitor binding pocket (mBio, 2019)
Molecular modeling applications
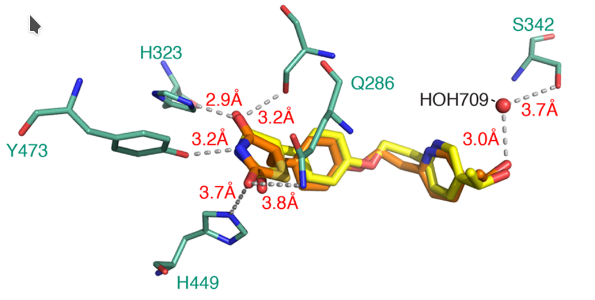
Application of molecular modeling techniques, like molecular dynamics, molecular pharmacophores, and virtual screenings.
Relevant publications
-
Structural basis of altered potency and efficacy displayed by a major in vivo metabolite of the antidiabetic PPARγ drug pioglitazone (J.Med.Chem, 2019)
-
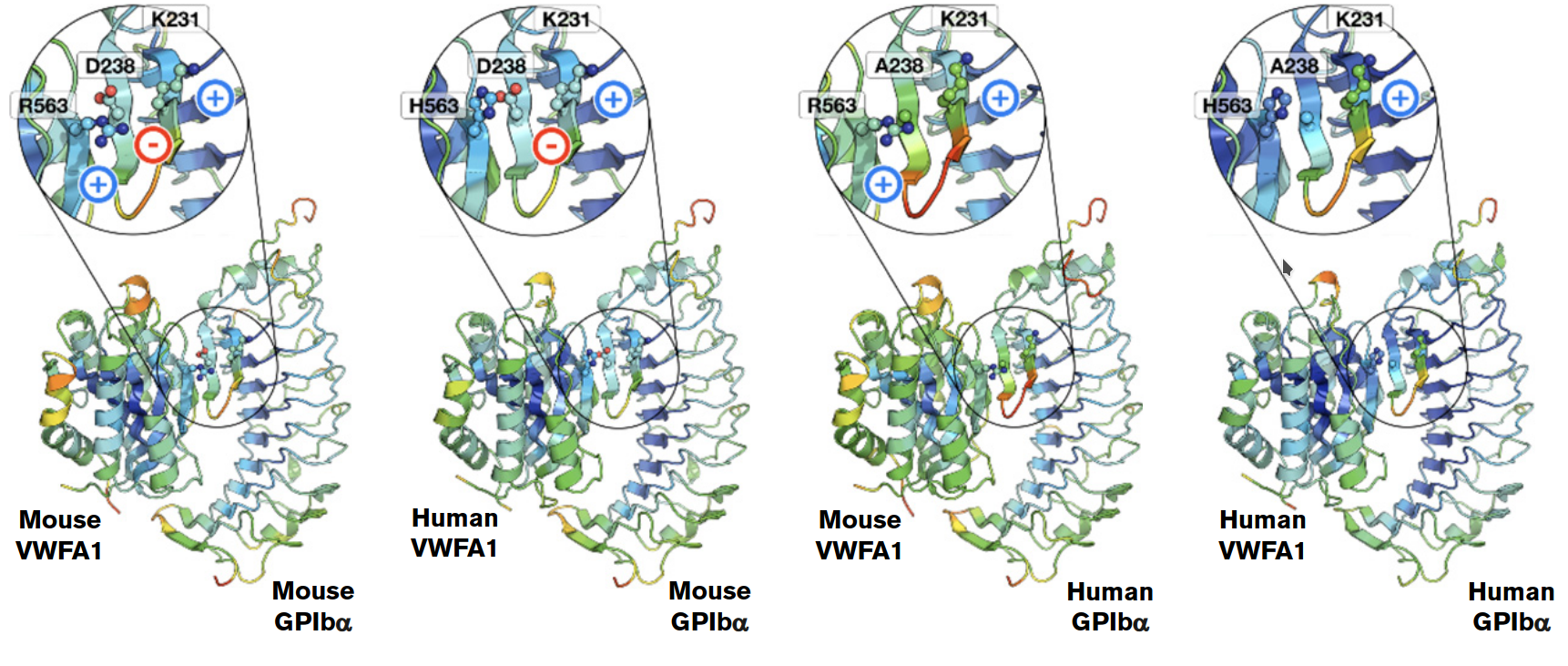
Humanized GPIbα–von Willebrand factor interaction in the mouse (Blood Adv., 2018)
-
Natural product anacardic acid from cashew nut shells stimulates neutrophil extracellular trap production and bactericidal activity (JBC, 2016)
-
Directional phosphorylation and nuclear transport of the splicing factor SRSF1 is regulated by an RNA recognition motif (JBC, 2016)